RESEARCH INTEREST
HALOGEN BONDING (XB) CATALYSTS FOR ORGANIC SYNTHESIS
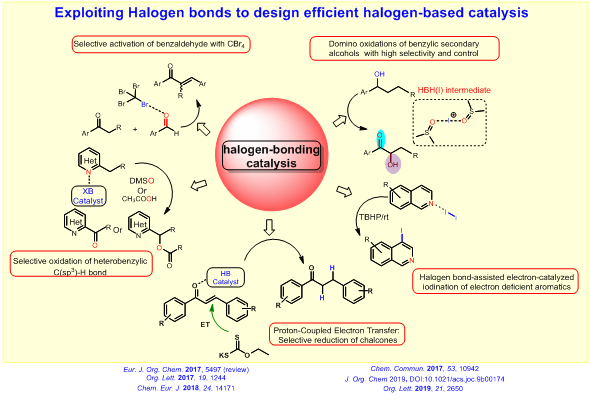
Our research group is mainly focused on halogen–bonding catalysis for organic reaction methodologies. Recently, halogen bondingcatalysts asorganocatalysts are employed to activate the Lewis basic functional groups. This XB interaction has been exploited tosynthesize recyclable catalysts, to photoactivatehalides to generate radical intermediates, and to transform gaseous compounds to easily–handleable condensed–phase liquid reagents.
Our primary research theme is to exploit this non–covalent XB interaction to design efficient halogen–based catalyst system. For this objective, we are trying to realize how the XB can influence the reactivity of a halogen (I) catalyst and intermediates in a reaction mixture. We investigate the feasibility of functional group activation by easily available halogen(I) reagent such as CBr4. The halogen bond can be employed to stabilize the otherwise unstable halogen(I) active species which can catalyze efficient oxidative methods. Recently, we have discovered that the XB can control the selectivity of oxidative functionalization of (aryl)(heteroaryl)methanes by halogen(I) reagents and can decrease the activation energy of electron transfer process. Also, the halogen–bond has been engaged as an efficient tool to enable an electron–catalyzedregioselective iodination of hetero aryls under mild reaction conditions. The halogen bond between hetero–aryl substrates (electron–donor) and iodine (electron–acceptor) lowers the activation energy of the electron–transfer (ET) from the former to the latter. Of late, other non-covalent interaction such as hydrogen bond has also been utilized for the selective reduction of chalcones and alkynes.
To figure out the general principle for designing halogen–bonding catalysis, we investigate the mechanism of these XB catalyzed reaction methodologies by employing advanced techniques of physical–organic chemistry such as NMR, UV–titration, IR mass spectrometric investigation, reaction kinetics analysis and quantum chemical calculation (DFT).
METAL NANOCATALYSTS FOR ORGANIC SYNTHESIS
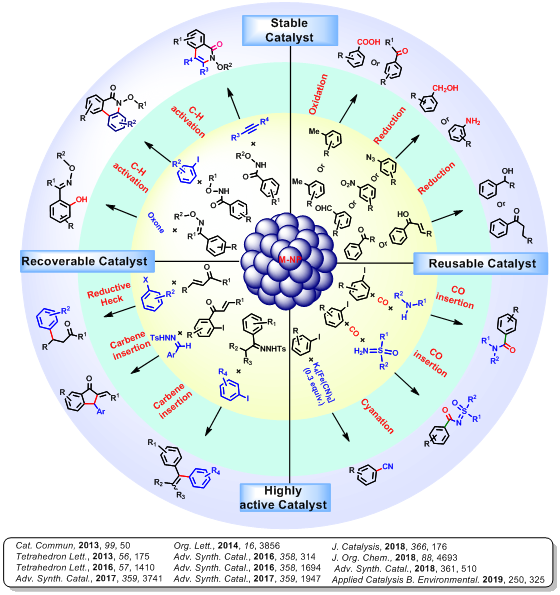
Most of the chemical reactions are mainly dependent on homogeneous catalysts as heterogeneous catalysts are less reactive. The reuse of precise metal catalysts (which is difficult for homogeneous catalysts) not only help the economy but also the environment by reducing its contamination by toxic metal. The drawbacks of both homogeneous and heterogeneous catalysts can be solved by using metal nanoparticles as catalysts. The catalytic activity of metal NPs is greatly determined by its size, shape, composition and stability. Our mainobjective is to synthesize highly stable metal NPs with an excellent catalytic activity. In this regard, we have designed and synthesized the binaphthyl stabilized NPs with average particle size 4.0 nm. The binaphthyl moiety stabilizes the NPs (Pd-BNP, Pt-BNP, Cu-BNP and Pd/Cu-BNP) through M-C covalent bond with metal centre. Presence of organic binaphthyl moiety in NPs, makes it semi-solubilize in organic solvents. After characterization of the synthesized NPs, it has been successfully utilized as an efficient catalyst for several organic transformations such as oxidation, reduction, cross-coupling, Reductive Heck, C-H activation, annulation reaction, etc.
DEVELOPMENT OF NEW SYNTHETIC METHODS USING N-TOSYLHYDRAZONES
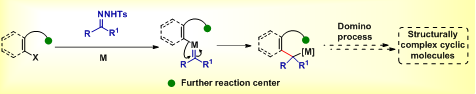
Our research activities focus on the development of alternative strategies to overcome the problems that arise with the handling of non-stabilized diazo compounds. In this context, we are exploring the intriguing reactivities of N-tosylhydrazones, a safe and stable carbene precursor in transition-metal catalyzed as well as transition metal-free transformations. Particularly, we are interested in engineering diverse domino approaches through carbene migratory insertion as a key step to construct structurally interesting and biologically relevant molecules. We are also interested in finding an application of our developed reactions in the synthesis of natural products.
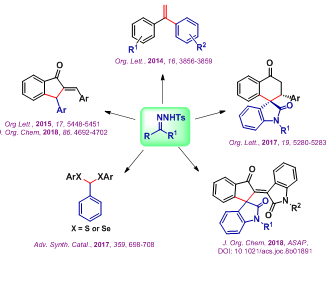
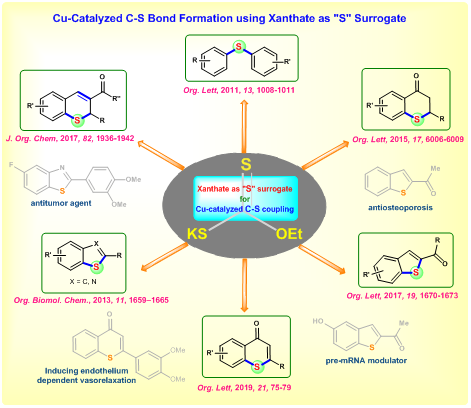
XANTHATE AS SULFUR SURROGATE FOR ORGANIC SYNTHESIS
Sulfur containing compounds are extensively found in various natural product and biologically active molecules. For instance, the top 10 best-selling drugs in 2012 contain sulfur. Also, sulfur compounds act as catalyst, ligand, oxidizing reagent and solvent in organic synthesis. Though sulfur compounds plays important role in diverse areas, synthesis of these molecules are limited compared to other hetero atom (N, O, P) containing organic compounds. One of the main reasons behind this is foul odor of the sulfur and nature of the available sulfur source. Metal catalyzed carbon-sulfur bond formation arerarely reported because of the metal catalyst poisoning by sulfur especially thiols and disulfides. These problems have been successfully overcome by various sulfur surrogates. In this regard, we have developed a copper-catalyzed C-S bond formation using xanthate as sulfur surrogate. Using this strategy, various methodologies have been developed to synthesize important sulfur containing cyclic and acyclic molecules through concomitant C-S / S-C bonds formation.
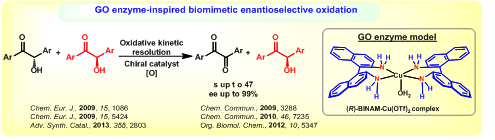
ENZYME MIMETIC CHIRAL CATALYSIS
For the first time, we have developed a chiral BINAM-Cu complex, as an enantiopure model of Galactose Oxidase (GO) enzyme, and utilized it in the efficient synthesis of important enantiopure alcohols such as benzoin, amino alcohols and a-hydroxy esters by oxidative kinetic resolution (OKR). This OKR is a very versatile and practical method as it uses molecular oxygen as the sole oxidant and produces only water as the by-product. This new chiral BINAM-Cu catalyst laid way for the development of several new chiral catalysts based on Co, Fe and Zn and various new enantioselective reactions such as enantioselective oxidative coupling, enantioselective coupling kinetic resolution and asymmetric hydrophosphorylation.
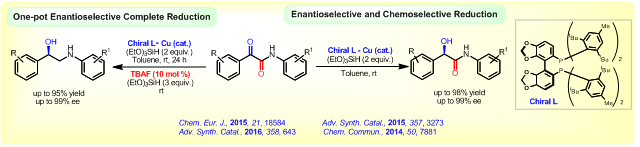
Also, we have demonstrated the BINAM-Cu complex (GO enzyme model) as an efficient catalyst for C(aryl)-N, C(aryl)-O, C(aryl)-S, and C(aryl)-C bond forming coupling reactions under mild reaction conditions.
RESEARCH HIGHLIGHTS
-
H-index: 35; Total citation: 3,758 (as on 25.06.2021; Source:Scopus; Scopus ID: 6602184820)
-
Our recent research regarding conversion of toluene to benzoic acid using Pt-BNP nano-catalyst in water (in Applied Catalysis B: Environmental, 2019, 250, 325 journals) is highlighted in “The Hindu” national English newspaper on 31stMarch, 2019 (Page 13 of Chennai Edition)
-
DD national (Science) TV / RS TV news coverage about conversion of toluene to benzoic acid using our Pt-BNP nano-catalyst in water (18.5.2019, Science Monitor)
-
ACS best Poster Presentation Award for our poster presentation at 24th CRSI-National Symposium in Chemistry(CRSI NSC-24) 8-10 February, 2019.
-
Best Poster Presentation Award for our poster presentation at 22nd CRSI-National Symposium in Chemistry(CRSI NSC-22) 2-4February, 2018.
-
Best Poster Presentation Award for our poster presentation at CCC-2013, CSIR-CLRI, Chennai, February 8-10,2013; Isolation and Characterization of Trinuclear Cobalt Complex in Secondary Alcohol Aerobic Oxidation Reaction.
-
Best Poster Presentation Award for our poster presentation at 14th Chemical Research Society of India National Symposium in Chemistry, February 3-5, 2012, NIIST, Trivandrum, India; Cu-catalyzed in situ generation of thiol and its application in synthesis of aryl thioethers, benzothiazoles and benzothiophenes
-
Our recent paper (Org. Lett., 2017, 19, 1244)is one of the most accessed top 20articles in Org Lett. for Month of FebMar, 2017.
-
Our paper (J. Org. Chem, 2009, 74, 3675) is one of the most accessed top 10 articles in J. Org. Chem. for the Months of April-June 2009.
-
Our paper (Org. Lett.2009, 11, 1923)is one of the most accessed 20 articles in Org Lett. for Month of April 2009.
-
The same research article has been featured in Synfacts, 2009, 8, 841 (Synthesis of 1,4-Benzoxazines by Domino SN2 and Goldberg Coupling).
-
Our recent paper (Chem. Eur. J. 2009, 15, 5424) has been featured in Synfacts, 2009, 8, 870 (Oxidative Kinetic Resolution of Racemic Benzoins).
-
Our paper (Tetrahedron Lett., 2009, 50, 2965) is one of the most accessed top 25 hot Science Direct articles for months of April-June 2009
-
Twenty three of our research articles have been featured in ChemInform
-
DABCO-CuCl complex synthesized and used by us for the oxidation of alcohols (S. Mannam, S. K. Alamsetti and G. Sekar, Adv. Synth. Catal., 2007, 349, 2253) is addedin ALDRICH catalogue with product no: 703141.
